GROWING INTERNATIONAL CONCERN OVER RAPID ANTIBODY TESTS AS THE WHO WARNS AGAINST THEIR USE AND INDICATES MOST DO NOT HAVE US FDA APPROVAL
Get our headlines on WHATSAPP: 1) Save +1 (869) 665-9125 to your contact list. 2) Send a WhatsApp message to that number so we can add you 3) Send your news, photos/videos to times.caribbean@gmail.com
(FLORIDA)We’re all enduring the COVID-19 outbreak, and we all want life to return to normal. But some say if we rush that process, it could spell trouble.
“Deaths and cases are on the upswing, so this is exactly the wrong time to be reopening the country,” said Dr. David Dodson, an infectious disease specialist in West Palm Beach.
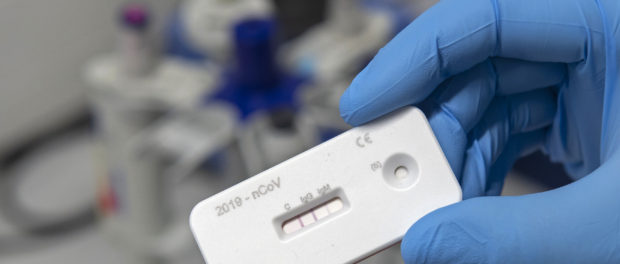
There are two types of testing being done right now – one, the swab jammed into your nasal cavity to see if you have coronavirus right now.
The other is a blood test that shows whether you have COVID-19 antibodies in your system to fight off the virus. If so, you may still be getting over the infection or have already had it and are no longer at risk of getting sick.
“The problem is a lot of these tests have not gone through the vetting process by the FDA so we don’t know whether they’re accurate or not,” Dr. Dodson said.
“There are many being sold on the internet that do not have FDA approval because they haven’t been studied well enough to say they accurately can detect whether you’ve had the virus or have the virus,” said Dr. Larry Bush, an infectious disease specialist and president of the Palm Beach County Medical Society.
Both experts we spoke with this afternoon raised concerns about the accuracy of tests currently available because they were approved under an emergency order, and didn’t go through the rigorous testing usually required by the FDA.
The danger, they say, is false positives could give us an inaccurate picture of whether ending social distancing is safe.
“The last thing they want to do is give a test out there that gives a false sense of security and false information,” Dr. Bush said.
According to published reports, about 90 companies—many based in China—have come out with these antibody tests that have not undergone proper FDA scrutiny.
“If you do it too early, we’re gonna have increased cases and increased deaths,” said Dr. David Dodson, referring to reopening the country.
Dr. Dodson says health officials need to take more time to look at these tests before allowing them to be used.
“I think the U.S. government is trying to reopen the economy too soon. I think you have to make a choice, whether you’re gonna preserve peoples’ lives or whether you’re gonna open up the economy,” Dr. Dodson said.
Leave a comment
You must be logged in to post a comment.